We often talk of the characteristics passed on from our families – be it our Dad’s hairline, our Grandmother’s nose or our Uncle’s musical talent. These characteristics can be influenced by our environment or by the genetic makeup we inherit. It is possible to quantify the effect of our genetic makeup on the ‘heritability’ of certain traits. This heritability is measured on a scale, where 0 means ‘no effect of genetics’ and 1 means ‘entirely due to genetics’.
Along with physical features and skills we also inherit a predisposition to certain diseases, including cancer. Observational studies have shown blood cancers to cluster in families (Figure 1), in particular clustering of multiple myeloma (MM) and chronic lymphocytic leukaemia (CLL) and this suggests a shared inherited susceptibility to these diseases. However, these studies cannot delineate the contribution from genetics and environment on disease risk.

Recently, our lab has performed a genetic correlation between MM and CLL which allows us to quantify the genetic component of shared inherited predisposition to the diseases. If the heritability of a disease can be thought of as a fingerprint of genetic variation within the genome that contributes to disease risk, then the technique we used to quantify the genetic correlation has sought to measure the similarity between the two ‘fingerprints’ of MM and CLL. We found a positive genetic correlation between MM and CLL supporting the observational studies and providing the first direct evidence of shared inherited genetic susceptibility to the diseases.
We found a positive genetic correlation between MM and CLL
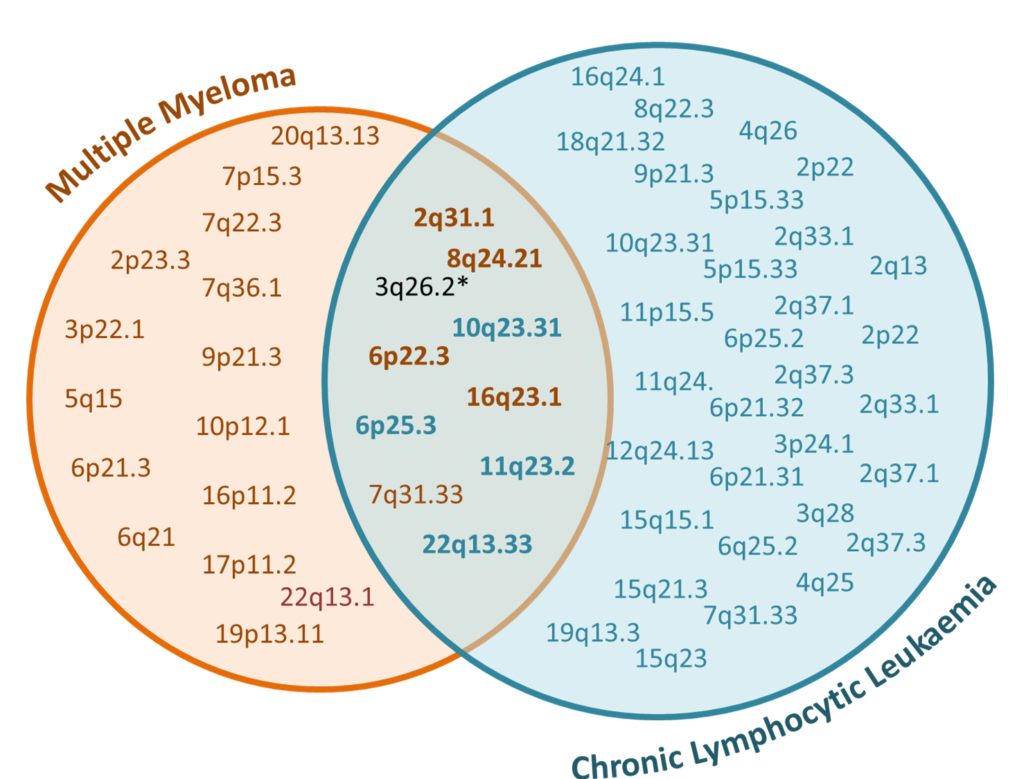
The ‘units’ we use to measure genetic correlation are single nucleotide polymorphisms (SNPs), which is a single base change in DNA. We look at the correlation in frequency and effect of these variants on the disease risk. The variation in frequency of SNPs have been investigated in genome-wide association studies (GWAS). Within the vast number of SNPs genotyped in GWAS, some regions of co-inherited SNPs (loci) are seen significantly more frequently in disease cases compared to controls. To date, there are 45 CLL and 23 MM risk loci. In addition to the discovery of a genetic correlation between these blood cancers, we also found further evidence of shared genetic susceptibility at 10 of the known risk loci (Figure 2).
The discoveries made in GWAS are robust to replication and the data we gain is valuable. From these risk regions we can quantify heritability, allowing us to deconvolute the influence of environment versus genetics on disease, and we can build polygenic risk scores, which enable us to stratify high risk individuals who may require more frequent or earlier screening for a disease.
our genetic code underpins the higher biology we observe
While treatable, there is currently no cure for MM and CLL and the aetiology of the diseases are still poorly understood. In addition to risk stratification, a further area of investigation for GWAS discoveries is the functional biology which underlies the association. This may give insight into mechanisms behind the disease. Common genetic variation, such as SNPs, localises to non-coding regions of the genome and are thought to influence disease pathogenesis through alteration of gene expression. To gain preliminary insight into the biology underlying MM and CLL, we integrated regulatory data from chromosome capture, histone marks and transcription factor binding at the 10 shared risk loci. Interestingly, we found genes at these loci which were implicated in B cell development, genomic stability and development of other cancers. This supports the tenet that these regions can influence the development of either disease, especially as MM and CLL originate from the same cell at different stages of maturation. In the future, we aim to investigate GWAS discoveries in a biological setting, providing in vitro and in vivo evidence of interacting genes and mechanisms of regulation.
Ultimately, our genetic code underpins the higher biology we observe, from cell behaviour to development and disease. As germline genetics are set at conception and are understood to be stable, they provide a robust foundation to understand the aetiology of disease. As such, studies of germline genetics offer the prospect of identifying genes and pathways perturbed in disease pathogenesis and identifying potential therapeutic targets.
Comments